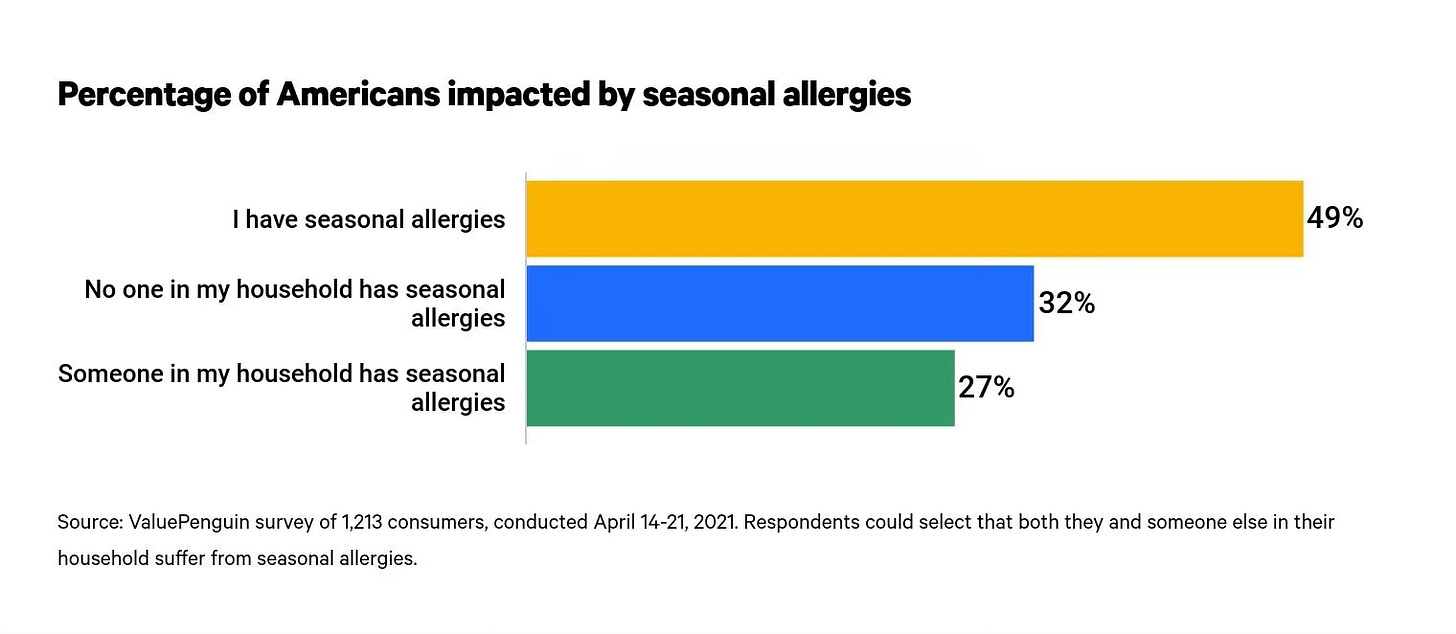
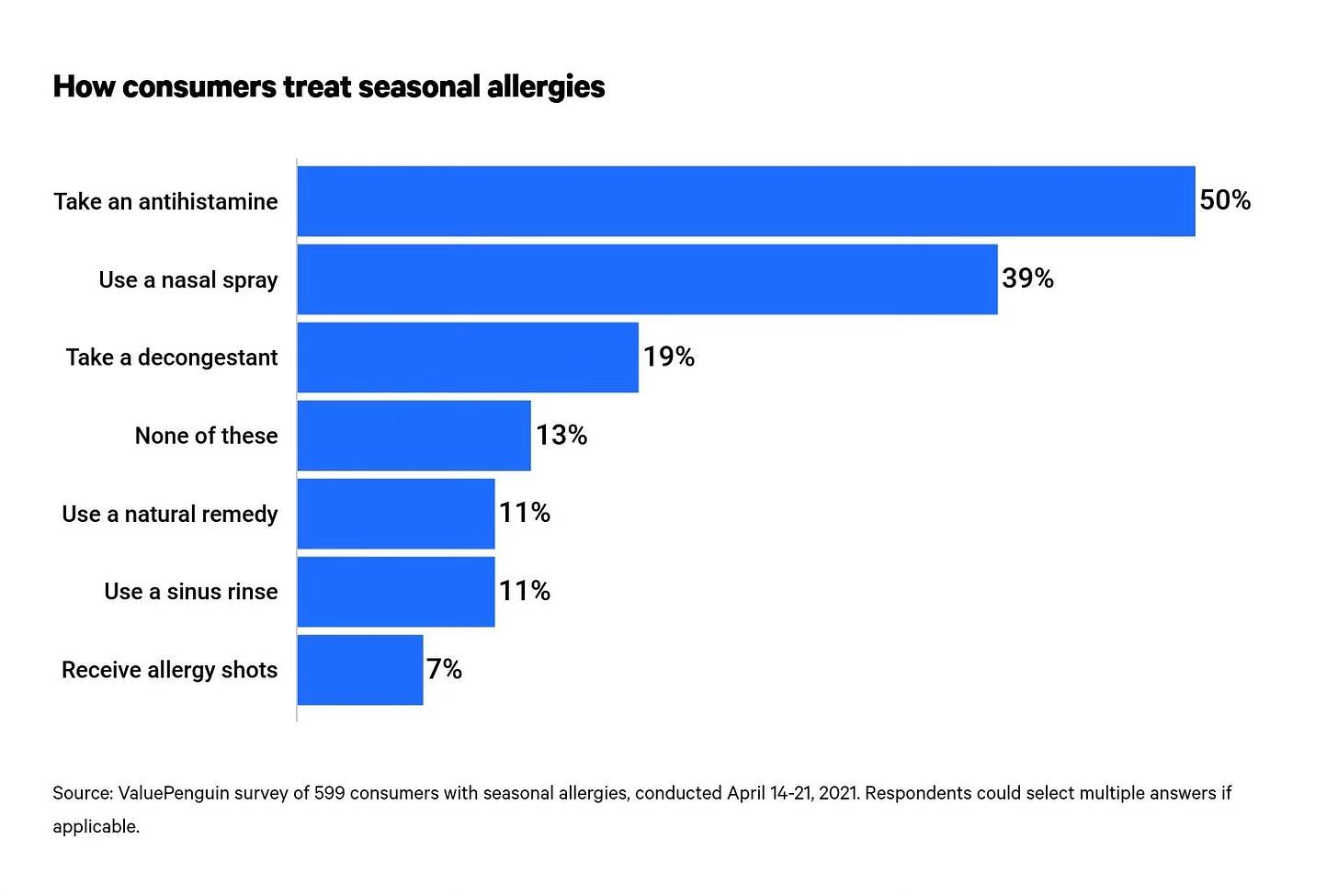
The arrival of spring, is a time of renewal. Greening of trees, blooming of blossoms, warmth and t-shirts. But it also portends a very less welcome guest for millions: seasonal allergies. As these blossoms bud, as the grasses grow they release pollen, and with that comes a cascade of uncomfortable symptoms for many people. For those who suffer, the remedies are limited with many relying on over-the-counter remedies.
And so here we are, after decades for which the primary defense against these airborne invaders has been antihistamines which (as the name suggests) work around the bodies immune system and its release of histamine. This is clunky and just provides relief, it doesn’t address the root cause of the allergic reaction. Furthermore, they often require daily and consistent use.
However, this landscape is on the cusp of transformation. A new season (ugh, sorry) of potential relief.
Monoclonal antibodies.
Lab-made proteins that can precisely target and neutralize the body's allergic response at its source. Stop the allergy, not the reaction to the allergy.
One such medication, omalizumab (AKA Xolair, and something that was initially developed for asthma) in particular has shown remarkable success in early clinical trials. These trails have shown that when administered as a single injection a couple of weeks before the allergy season typically begins, recipients experience months of relief! A stark contrast to the daily vigilance required by nose sprays and other traditionals.
The mechanism is elegant. It’s stopping the rain while in the clouds. The monoclonal antibodies primarily targets immunoglobulin E (IgE) in the patient’s bloodstream, immunoglobulin is the antibody that triggers the allergic cascade. Without the shot, when IgE detects an allergen, it binds to immune cells and instructs them to release histamine and away the body goes with its inflammation and congestion. With the binding part of this reaction inhibited, the allergy and its system is rendered reactionless. Its symptoms never occur.
There’s more good news as the viability of the monoclonal antibodies has the potential to address a wide spectrum of allergic reactions concurrently. Clinical associate professor at Stanford University, Sayantani Sindher, highlights:
“<These therapeutic drugs> offer the potential to target the underlying pathways driving all allergic reactions in general,"
What he means is that we may be looking at Spring seasons where meaning a single treatment could alleviate both seasonal and food allergies both!
The FDA has already approved omalizumab for treating food allergies, but the journey to widespread adoption of these cutting-edge treatments is not without its hurdles. Currently, the cost of omalizumab is prohibitively high, which limits its use and adoption potential. But a generic version has been approved, and we may see prices decrease. Outside of the USA, large-scale clinical trials also have shown very promising results offering a near future with more monoclonal antibodies on the market and prices facing another downward pressure point.
High development costs are a problem with modern medicine, but they come with miraculous discoveries and wonderful positive change. It’s a tough pill to swallow (ugh) but I, for one, will take the pharmaceutical industry at the forefront of medical innovation.
As the advancements in monoclonal antibody therapies for allergies show, good research and development will continue to yield remarkable treatments.
Consistently pushing the boundaries of science to create groundbreaking medicines is, and will always be, a good thing.